
A recent WhatsApp message presented a series of arguments discrediting the claimed 95% efficacy of Pfizer vaccine. The message is partly correct, but it is misleading people by claiming that the clinical trial was conducted on a selected group of participants aimed for optimal results.
By Pankhuri Agarwal
A recent WhatsApp message being widely circulated in India claims that 95% efficacy of Pfizer vaccine is a “BIG LIE” and “the calculations included extremely complex mathematics which only creates confusion.” However the press release by Pfizer states that the primary efficacy analysis demonstrates BNT162b2 to be 95% effective against COVID-19 beginning 28 days after the first dose.
Below is the screenshot of the message which is partly true but misleading people with complex language and explanation of the calculation.

Vaccine efficacy is used when a study is carried out under ideal conditions, for example, during a clinical trial; and vaccine effectiveness is used when a study is carried out under typical field (ie, less than perfectly controlled) conditions, as stated by the Centre for Disease Control and Prevention (CDC).
The second part of the message says, “Vaccine efficacy is calculated under optimal conditions, excluding elderly and immunocompromised participants.” This is false.
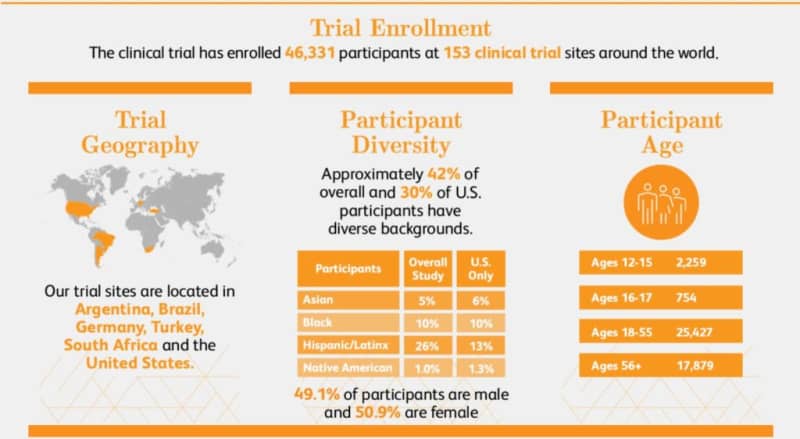
The BNT162b2 mRNA COVID-19 vaccine study states that the triple-blind phase 2/3 trial was conducted at 153 sites globally, with 46,331 participants – 97 percent of them were aged 16 years or older.
A breakdown of the diversity of participants is publicly available.
The number of participants with comorbidities according to the Carlson Comorbidity Index is also publicly available.
We reached out to experts to know more. Dr. Siddharth Sridhar of The University of Hong Kong said “the Pfizer trial was a multinational trial including a representative swathe of the general population including many elderly individuals. So the claim that ‘in all trials they had super fit, superhuman type individuals like non-drinkers, non-smokers and highly active gym nuts’ is simply false.”
Moving on to the third part of the WhatsApp message, it misleadingly explains the calculations, leaving out critical information.
Analysis of the study data indicates a 95% vaccine efficacy rate in participants without prior COVID-19 infection and also in participants with and without prior COVID-19 infection.
“Furthermore, real-world efficacy data from Israel shows that the vaccine effectiveness of the Pfizer vaccine is almost identical to the vaccine efficacy calculated in the phase III trial. They have published this data in the New England Journal of Medicine,” added Dr. Sridhar.
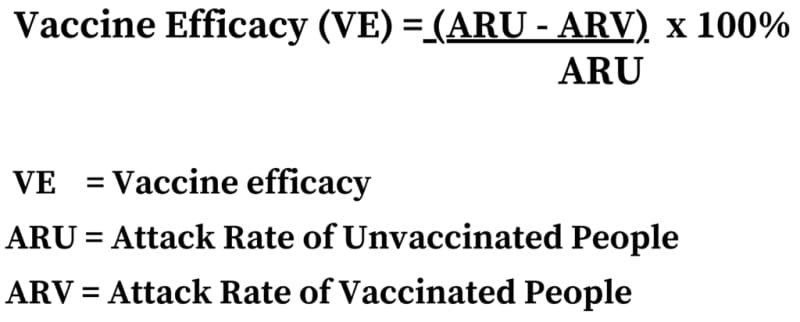
To calculate the vaccine efficacy in the BNT162b2 mRNA vaccine clinical trials, the participants were studied in two groups – Main Safety Subset, with 37,706 participants; and the Modified Intention to Treat (mITT) group, with 43,355 participants vaccinated with Dose 1.
The vaccine efficacy was calculated for four Efficacy Endpoint Subgroups – At Risk, Age group (years) and at risk, Obese, Age group (years) and obese. 95% Confidence intervals (CI) were then derived based on the Clopper and Pearson method, adjusted for surveillance time.
Therefore, the primary efficacy analysis demonstrates the Pfizer vaccine to be 95% effective against COVID-19 beginning 28 days after the first dose.
The post Misleading WhatsApp message questions Pfizer vaccine efficacy appeared first on Health Analytics Asia.
